This is part 4 of a 1, 2, 3, 4 part series on Regulation of Genetically Modified Animals
I am in perhaps a somewhat rare position regarding the contemplated USDA regulatory framework, as I actually have several animals from amenable species (sheep, cattle) on the ground that were developed using “techniques that use recombinant, synthesized, or amplified nucleic acids to modify or create a genome” for “agricultural purposes”. I have not published these data and they are the dissertation research projects of graduate students, and so I will only say that these animals were produced using CRISPR/Cas9 genome editing and a sgRNA to produce a gene knockout. This was done by introducing a Cas9-sgRNA ribonucleoprotein (RNP) into single cell zygotes following in vitro fertilization of in-vitro matured oocytes. The resulting double strand break (DSB) was repaired by the non-homologous end joining (NHEJ) pathway, and subsequent embryo transfer to surrogate dams. In other words, each animal is a unique individual, and there was no foreign template or intergeneric combination of genetic material introduced into the editing process at any time. None of the genome editing target genes were intended to impact animal health, nor was the application intended to have an animal health claim. So as I read through the contemplated regulatory framework, I cant help but personalize how it would affect my research, and how it compares to the existing regulations.
By way of background, it is perhaps important to emphasize that the animals that make up the research herds and flocks at land-grant universities are typically entering the food supply at the end of experiments. Our dairy cattle getting fed different experimental rations keep getting milked, the calves from a beef cattle crossbreeding study are finished in our feedlot and enter commerce at the end of the study, and our poultry produce eggs that are sold to the public, along with meat products from all our animal facilities (except the horses!). There we sell foals at the UC Davis foal auction. The food animals are actually processed at our USDA-inspected slaughter facility, and butchered by meat science students. The income from the “meat lab” sales goes back into the operational account for the animal facilities.
So back to the genome-edited knockout animals I have on campus. Under the existing FDA regulations, they are considered unapproved animal drugs and cannot enter the food supply without a new animal drug approval or prior authorization. According to the FDA, I would need to apply for an investigational new animal drug (INAD) for these animals, and then request a Food Use Authorization (FUA) for these experimental animals to enter the food supply at the end of the experiment. I have actually already started to apply for that and the FDA have requested an answer to the following questions before they can even open an INAD, let alone tell me what information I need to provide to them to potentially obtain a FUA for my experimental gene knockout sheep and cattle. I have started on this paperwork, but it will take some time to prepare it all and submit it through their electronic portal.
- Description of the project, including the goal of the intentional genomic alterations (IGAs), any relevant information from other animals in which the same genes have been knocked out (e.g., references to published articles characterizing knockout mice), and a description of the planned studies
- Description of how the IGAs were produced, including detailed experimental design
- Include information such as which Cas9 nuclease variant was used (e.g., SpCas9, SpCas9-HF1) and any associated information regarding the variant’s fidelity, criteria used to design and select guide RNAs, and any controls utilized to mitigate the potential for unintended alterations
- Available molecular characterization data, including a description of the methods used (e.g., PCR, Sanger sequencing, long-range PCR, etc.)
So under the USDA contemplated regulatory framework, published in the Federal Register 12/28/2020, these knockout animals would clearly fit under the “amenable species modified or developed using genetic engineering and intended for agricultural purposes and human food”. If they were knockout plants they would have an up-front exemption from regulation under the SECURE revision as they “result from natural cellular repair of a targeted DNA break without any introduced DNA to direct the repair”.
For the USDA animal health safety review I would need to show that “the animal under review is found to pose no greater risk to animal health than the animal from which it was derived”. This is where is gets a little unclear. The term “the animal from which it was derived” suggests that the animals are being derived from a known unmodified animal, presumably through genetic engineering of a cell line derived from that animal, followed by somatic cell nuclear transfer cloning of the edited cells. But my animals are derived from editing multiple zygotes, so each edited animal has its own unique genomic sequence i.e. there is no animal from which these animals were derived, and conceptually editing outcomes differ between all of these different individuals, especially because the DSB was repaired using the error-prone NHEJ pathway. So does each individual animal have to have a different evaluation?
The safety review requires “a molecular characterization of the modification and an understanding of the process by which it was introduced, that the intended change was made and that there were no unintended disruptions of endogenous genes, unintended DNA insertions, or off-target changes if the genome was modified without inserting DNA.” It is not hard to prove that the intended change was made, we typically do that with a PCR amplification of the target locus, and sequencing of the amplified PCR product. But to answer the rest of those questions will require, at a minimum, whole genome sequencing of every animal, and proving a negative i.e. proving no unintended disruptions of endogenous genes, unintended DNA insertions, or off-target changes if the genome was modified without inserting DNA. Both the sequencing and the bioinformatics to try to answer these questions would be very expensive, well beyond the cost of a sheep, or even a cow. This is especially true when every animal has a unique genotype, and there is no way to differentiate off-target changes from spontaneous de novo mutations.
Further, at a minimum, the animal health risk assessment would include an evaluation of the following issues:
- Molecular Characterization: What is the genetic modification(s) in the animal, how was the genetic modification(s) introduced, and how does the genetic modification(s) alter protein or ribonucleic acid (RNA expression)?
- Animal Health: Is there scientific evidence that the modified animal could plausibly, either directly or indirectly, increase susceptibility of livestock, including of the animal itself, to pests, non-infectious diseases, or infectious diseases of livestock, including zoonotic diseases? Is there scientific evidence that the modified animal could plausibly increase the spread of pests or infectious diseases of livestock, including zoonotic diseases? When a plausible pathway to such an increased risk is identified, further analysis would be conducted to evaluate the pathway. When an animal health claim is made or a modification is known to adversely affect animal health, the review would assess the animal health claim.
- Environmental Factors: Is there scientific evidence that introduction of the modified animal into the environment may result in environmental impacts that would warrant review pursuant to the National Environmental Policy Act (NEPA) or other statutes?
And in addition, the FSIS review would include an evaluation of the following issues (theoretically pre-slaughter, although obtaining meat for compositional analysis from animals pre-slaughter is obviously problematic):
- Evaluation of expressed substances: Is there scientific evidence that the genetic modification could result, directly or indirectly, in toxins, chemical residues, or other potentially deleterious substances in meat or poultry products?
- Allergenicity: Is there scientific evidence that the genetic modification would directly or indirectly alter the allergenic potential of meat or poultry products derived from the animal?
- Food storage and processing: Is there scientific evidence that meat or poultry products derived from the modified animal could mislead consumers regarding wholesomeness or the need for appropriate storage (e.g., meat that maintains a red appearance even when spoiled)?
- Compositional analyses of key components: Is there scientific evidence that meat or poultry products from the modified animal are compositionally (e.g., nutritionally or functionally) no different than meat from conventional animals, such that it meets any regulatory definition, standard of identity or other labeling requirement, and consumer expectations for the applicable product?
Needless to say, providing answers to all of these questions for each individual knockout animal would be an unsurmountable hurdle for an academic laboratory. Throughout the USDA contemplated regulatory framework the term “developer” is used. For example, “Under the contemplated regulatory framework, developers could request that USDA conduct a risk-based and science-based safety review focused on animal health”. Historically, developers of genetically engineered plants have been large multinational companies who have the resources to provide the regulatory data to support commercialization and ultimately deregulation of their products. Research universities are not developers in this sense, but we still produce amenable species modified using genetic engineering and intended for agricultural purposes and human food.
One of the questions the USDA poses in the request for comments is,
“How often does a start-up company or not-for-profit university or research organization modify or develop an animal using genetic engineering?”
My answer would be never. Researchers at universities often modify animals using genetic engineering but never has one developed and commercialized an “amenable species modified or developed using genetic engineering and intended for agricultural purposes and human food.” The two food approvals for genetically engineered animals were both obtained by companies, albeit small companies. AquaBounty for the “AquAdvantage” salmon, and Revivicor for the “GalSafe” pig. To date neither of these products has been sold for food in the United States, although AquaBounty is getting mighty close.
In closing, as long as animals produced using genetic engineering, even those that could have been produced using conventional breeding, are subjected to unique regulatory scrutiny not required of identical products produced using conventional breeding, research in food animals using genetic engineering for agricultural applications will be cost-prohibitive in the United States.
Another question the USDA poses is,
“Should USDA exempt certain types of genetic modifications of amenable species intended for agricultural use from regulation? If so, what types of modifications and why?”
I would suggest that the SECURE up-front exemptions for certain types of modifications in plants, specifically a modification (1) resulting from natural cellular repair of a targeted DNA break without any introduced DNA to direct the repair; (2) that is a targeted single base pair substitution; or (3) that introduces a gene known to occur in the plant’s gene pool, or causes a change in a gene that corresponds to a version of that gene present in the organism’s gene pool should be extended to genetically engineered animals. These exemptions were primarily designed to exclude from regulatory oversight organisms that could have been achieved using conventional breeding. Just like the Coordinated Framework calls for.
If you have comments on the contemplated regulatory framework, there is a 60 day public comment period that closes 2/26/2021. I would encourage all people working in this field to post comments. Comments can be posted Federal eRulemaking Portal: https://beta.regulations.gov/comment/APHIS-2020-0079-0005. Go to Supporting documents and any comments that are received on this Advance Notice of Proposed Rulemaking may be viewed at https://beta.regulations.gov/comment/APHIS-2020-0079-0005.
In the words of one commentator
“It, of course, remains to be seen what will be the Biden Administration’s approach to ag biotech; however, given the consistency of approach to the evolving ag biotech regulatory structure by both the Obama and Trump Administrations, it would not be surprising if the Biden Administration also continued the evolution of a more science-based, evidence-based, and risk-based approach to regulation of ag biotech – including genetically engineered animals. It would behoove stakeholders that would support such consistency of regulatory approach to submit detailed comments that would assist USDA in developing such a notice of proposed rulemaking (NPRM).
As with all opportunities to provide comments on Federal agency proposals, it is important to submit concise, substantive, and well-supported and well-documented comments to the administrative docket. In this case, given the trans-Administrations nature of the action, it is important that interested stakeholders use this opportunity to best advantage to urge the Biden Administration to continue a 21st Century approach to regulating ag biotech.” Keith Matthews, Wiley Rein LLP
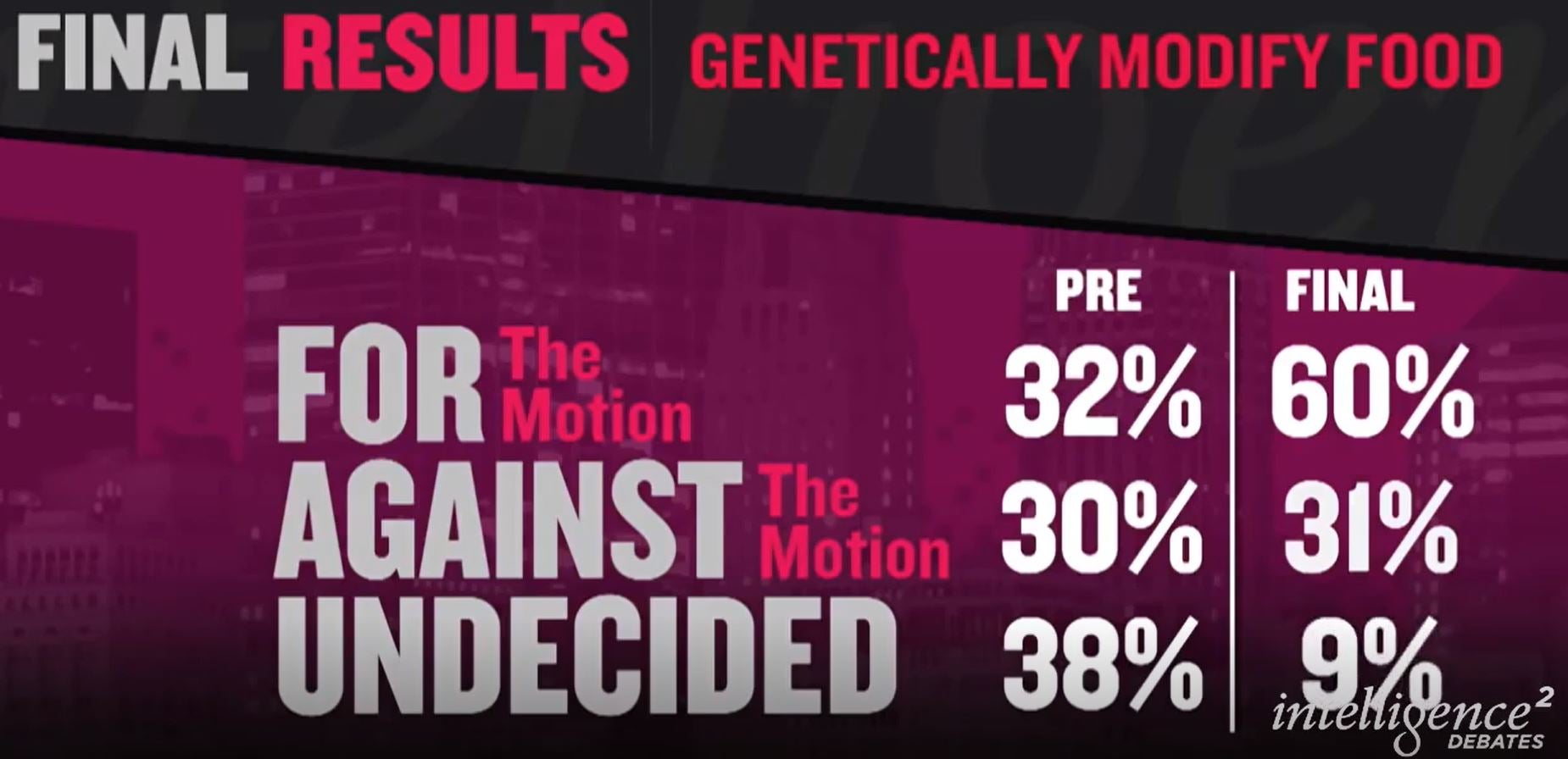
 Ten years ago on December 3, 2014, I participated in an Intelligence Squared (IQ2) debate in the Kaufmann theater New York City. The topic was “Genetically Modify Food”, and unexpectedly following the 90-min debate the side arguing for GMO Food, myself and Monsanto executive vice president and chief technology officer Rob Fraley, swayed a large proportion of the New York city audience to vote “YES” for GMO food. If you want to watch the whole 1 hr, and 43 min debate it can be seen at the bottom of the following web page https://opentodebate.org/debaters/alison-van-eenennaam/
Ten years ago on December 3, 2014, I participated in an Intelligence Squared (IQ2) debate in the Kaufmann theater New York City. The topic was “Genetically Modify Food”, and unexpectedly following the 90-min debate the side arguing for GMO Food, myself and Monsanto executive vice president and chief technology officer Rob Fraley, swayed a large proportion of the New York city audience to vote “YES” for GMO food. If you want to watch the whole 1 hr, and 43 min debate it can be seen at the bottom of the following web page https://opentodebate.org/debaters/alison-van-eenennaam/