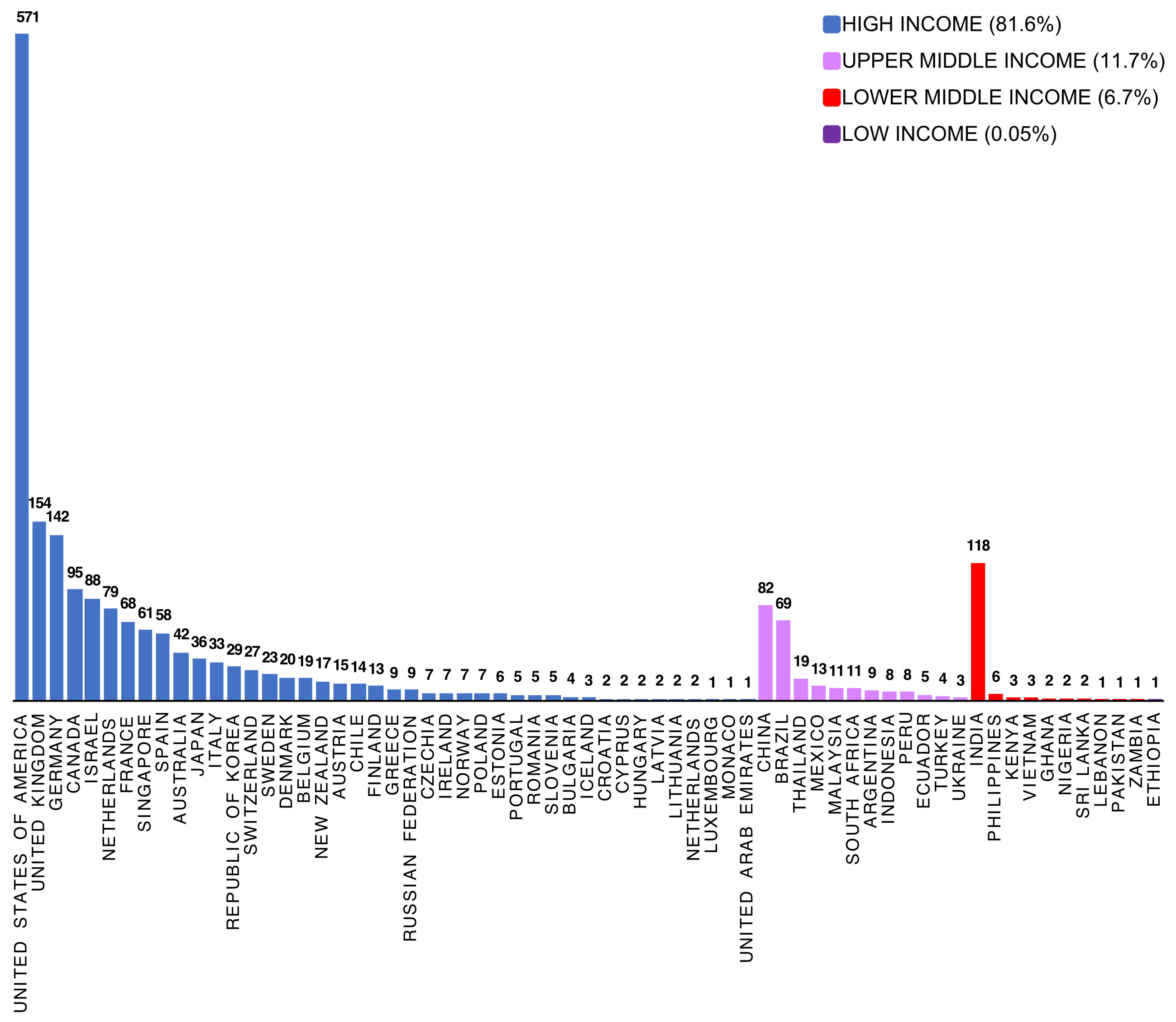
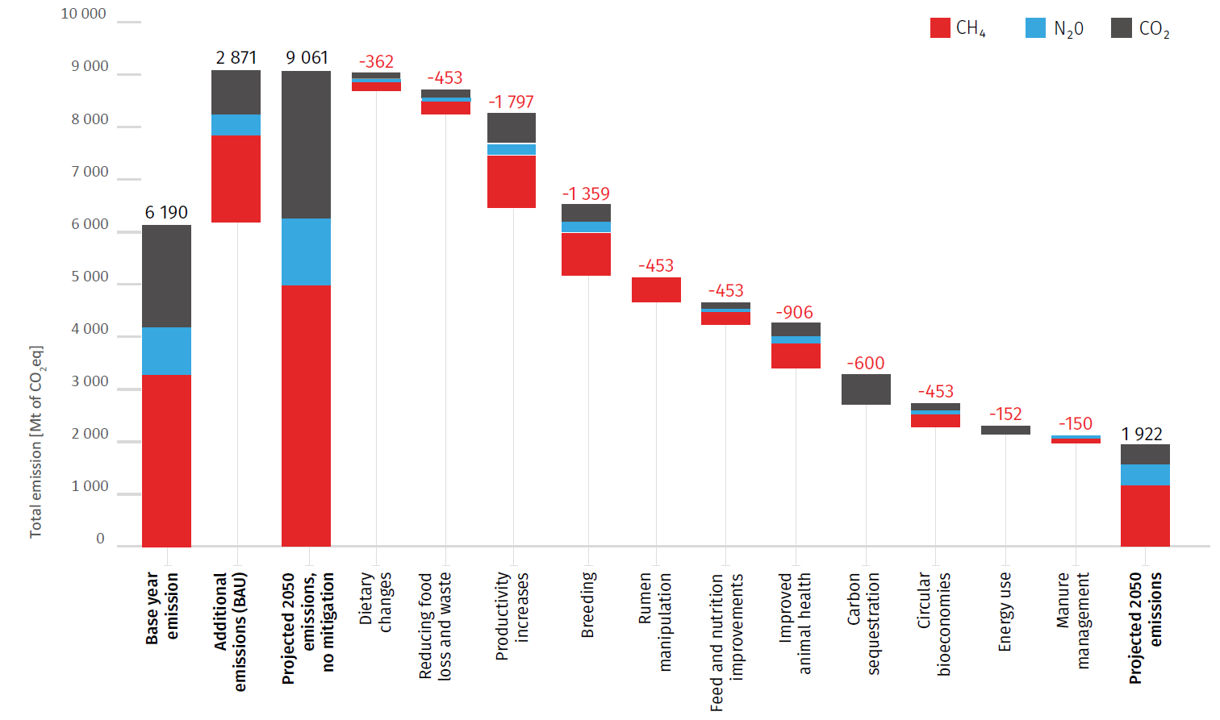
Animal biotechnology is the application of modern molecular techniques to animals. Genetic engineering and cloning are two older forms of animal biotechnology , and genome editing is a more recent entrant. Animal genomics is the scientific study of structure, function and interrelationships of both individual genes and the genome in its entirety. Utilization of genomic information in breeding is often referred to as genomic selection (GS). In my view these two fields – biotechnology and genomics – face entirely different public acceptance issues. I wrote a conference proceedings paper for a forthcoming conference in November 2021, and drew on some of my work in the past in this area. However the “My thoughts” section of the paper is new and published here for the first time. The entire paper (which is not yet peer-reviewed) is available on my laboratory website .
My thoughts
There exists a considerable literature castigating “scientists” (typically meaning research professionals and bench practitioners) for poor communication with the public on the topic of genetic engineering and cloning, and more recently genome editing and GS. The contention seems to be that this failure to communicate uncertainty is what historically “provoked public alienation and fomented controversy” around these technologies, and that this will likely cause problems for genome editing and GS. I beg to differ. Unless these later two topics become politicized, or perhaps more importantly competing business interests develop an approach to monetize fear around these technologies by inflating public perceptions of risks and arousing opposition in an attempt to trigger a spiral of silence (Scheufele, 2014), they will be integrated into livestock breeding programs largely without public scrutiny in the same way as other breeding advancements have been. Artificial insemination has not been recently communicated to the public, and yet its use is routine. However, if they become targeted, both bench and social scientists will have a hard time being heard above the drone of misinformation on social media where science and politics are inextricably linked, similar to what we observed with communications around uncertainties and relative risks associated with COVID vaccines and treatments.
I use the following evidence and observations to support these assertions. There is no money to be made opposing GS. There is no “Non-GS Project” label. There are no large multinational companies controlling its use that can be used as a proxy for evil (e.g. Monsanto). I do not foresee a targeted campaign to preclude the use of GS in genetic improvement programs, in part because it is founded on naturally-occurring genetic variations, and in part because it is hard to problematize into a clean, dichotomous framing i.e. genomic bulls are “bad”, and conventionally-selected bulls are “good”. And while many of the same criticisms leveled against GE and cloning can be equally associated with GS (e.g. increasing the rates of inbreeding), these concerns are likewise associated with conventional selection programs.
Artificial insemination reduces genetic diversity, and conventional selection programs include traits like docility which could be considered a behavioral disenhancement. Layers are selected to not exhibit broody behavior. I am unaware of any campaigns to preclude the incorporation of temperament traits into breeding goals for ethical reasons, despite the fact this clearly alters the telos of the animal. Additionally, there are glaring disparities when it comes to the implementation of GS in the developing world, and even in small breeds; it is expensive to develop large populations of genotyped, phenotyped animals. It is not a scale-neutral technology, advantaging large breeds and genetic providers over small ones. Such inequality concerns would be problematic for a GE application, yet these concerns are rarely even discussed as it relates to GS, and they have not precluded the adoption of this technology. Genomic selection is not a perfect science, there are uncertainties and emerging issues (Misztal et al., 2021), but it is the most accurate tool we have to select the future performance of the offspring of an individual. The absence of an additional regulatory layer to the use of genomic testing has allowed the unfettered, uncontested and rapid adoption of GS in livestock breeding programs globally.
Cloning is clearly unnatural, well at least somatic cell nuclear transfer (SCNT) cloning is unnatural in that it takes place in a laboratory. Cloning is actually rather common in nature, as evidenced by identical twins. Cloning elite animals has no obvious benefit to the consumer, and really is not that useful in breeding programs as it replicates the current generation rather than the next generation. It has had limited application in serving as a genetic insurance policy, and at times enabling the production of elite sires using less resources (Kasinathan et al., 2015).
By these metrics it would appear cloning is destined for market failure. And it has been effectively banned in the EU. In the Netherlands, the Dutch Animal Health and Welfare Act and Animal Biotechnology Decree prohibited the application of biotechnology to animals without a specific license. Criteria for being given a license included: the goal serves a public interest, has no unacceptable impacts on health and welfare of animals and does not raise any overriding ethical objections. It is characterized as a ‘No Unless’ policy – no application of biotechnology to animals unless there is a very good reason for doing so. Since 2005, Denmark has required special licensing for animal biotechnology through the Act on Cloning and Genetic Modification of Animals. This legislation came about in large part due to ethical concerns surrounding the impact of biotechnological applications on animal integrity. This Act effectively limits the commercial use of animal cloning and genetic engineering to “creating and breeding animals producing substances essentially benefitting health and the environment”.
However, in countries where cloning is allowed, opposition to cloning has slowly faded, and it is being adopted where it is cost-effective – mostly in high-value recreational animals like bucking bulls and polo ponies. I would argue in countries where clones are not regulated differently to conventional breeding, and products from clones are not labeled as they are in fact impossible to differentiate from products from non-cloned animals –(despite the apparent green milk moustache in Figure 1!), there has been no way to effectively monetize fear around clones.

Figure 1. The Center for Food Safety depiction of cloned milk from their 2007 campaign against animal clones.
The Center for Food Safety, Consumers Union, Food and Water Watch, The Humane Society of the United States, the American Anti-Vivisection Society, the Consumer Federation of America and the Organic Consumers Association tried hard in the early days of cloning, but at the end of the day it is hard to create a convincing argument that a cloned product is somehow more dangerous than its identical progenitor. And in the absence of tracking or labeling requirements, it was just not possible to create a cost-effective “absence-labeling” campaign as was done with rBST and GMOs.
It is worth noting that a lucrative pet cloning industry has emerged in the absence of regulatory oversight of non-food applications of cloning. In fact, Barbara Streisand recently took on two puppies cloned from her dead dog for the fee of $50,000. If there is a direct benefit, at least in the mind of the person cloning their pet dog or bucking bull, then people are willing to overcome their hesitations regarding cloning. And as to the entry of these clones into the food supply, it is mostly a moot point. Undoubtedly products from cloned livestock – elite breeding stock at the end of their productive life, and even bucking bulls at the end of their bucking career have entered the food supply on a limited scale. And considering that the US exported 190 million dollars’ worth of bovine semen in 2018, it is more than likely that there are offspring of clones running around globally.
And so we come to genome editing, the new kid on the block. And its fate is currently uncertain. Public perception is still forming around this technology, but I have a sinking feeling that genome editing will suffer the same fate as GE animals for the following reasons. Firstly, competing market forces have already started to conflate the two technologies. The Non-GMO project has come out with the following announcement “GMOs are now being created with newer genetic engineering techniques, some of which do not involve transgenic technologies. The Non-GMO Project is committed to preventing these new GMOs from entering the non-GMO supply chain.” The National Organic Standards Board voted to exclude all genetic modification and manipulation from organic production in 2016, including genome editing. And Greenpeace in their 2021 position paper entitled “Danger Ahead. Why genome editing is not the answer to the EU’s environmental challenges”, warns that the use of so-called gene (or genome) editing techniques like CRISPR-Cas could not only exacerbate the negative effects of industrial farming on nature, animals and people, but it could effectively turn both nature and ourselves (through the food we eat) into a gigantic genetic engineering experiment with unknown, potentially irrevocable outcomes.“ And so we again have a situation where activist groups and the natural and organic food industry will monetize fear and run a campaign of misinformation to suggest that genome edited animals are “unsafe”, whilst animals with naturally occurring genetic variants are “pure” (and also more expensive!).
Secondly, irrespective of the nature of the genome edit, the proposed regulatory approach to genome edited animals is the same as for GE animals, in both the EU and the United States. Even SNPs and deletions are being treated as drugs in the US. The absence of one intentionally altered base pair among 3 billion in the bovine genome thus results in an unsaleable new animal drug. By capitulating to this regulatory logic and tacitly agreeing that the emperor is wearing clothes, we replicate the situation where only large companies will be able to afford the regulatory and IP costs of bringing a genome edited animal product to market. Hitherto, the IP in livestock breeding has been primarily protected by secrecy and use of cross-breeding (Bruce, 2017). Small companies and academic laboratories will be unable to make use of a technology that originally resulted from public research funds. They will again be relegated to the sidelines, unable to afford even experimental work in large animals as all milk, meat and eggs from all genome edited “investigational animals” are unsaleable, and the animals themselves have to be composted, buried, or incinerated. There is then little incentive for public sector scientists to stick their neck out doing public communication around a technology they cannot use. Especially when doing so will likely result in hostile freedom-of-information act requests, and reputational defamation by front groups financed by the natural and organic food industry such as U.S. Right To Know (Kloor, 2015).

Figure 2. There are four species of transgenic fluorescent GloFish® available in six colors. Photo courtesy GloFish.
At the end of the day, I am not convinced widespread public opposition is what is preventing the adoption of new animal biotechnologies. The prevailing narrative repeated verbatim is that the public outright rejects GMOs. But that is not observed in actual purchasing behavior when GMO products are available. For example, GloFish® (Figure 2) are marketed to aquarists in the US, where they are now sold in every state in the nation, as well as throughout Canada. Sales represent approximately 15% of US aquarium fish sales. Although some authors raised early environmental and ethical concerns about GloFish (Rao, 2005), these concerns have waned over time.
GloFish is subject to enforcement discretion in the US. This is not a determination of “safety” under the Federal Food, Drug, and Cosmetic Act but is instead a determination that, based on risk, FDA does not believe it would be a good use of its limited resources to act against sponsors for the marketing and distribution of these unapproved products. Its sale is prohibited in other jurisdictions, including Europe, Australia, and Singapore. The success of this product suggests that consumers are willing to purchase GE animals, at least as aquarium pets. Alan Blake, CEO of the company marketing GloFish, wrote regarding public acceptance that consumers will purchase a product that they desire, irrespective of the breeding method that was used to produce it. In his words, “It is not about the process [of genetic engineering], it is about the product” (Blake, 2016).
Similarly, the Impossible Burger, a soy-based food product is proudly GMO with it recombinantly produced, bleeding leghemoglobin, has been a market success. Ironically the same anti-GMO groups that targeted GE in agriculture; GMO Watch, Consumer Reports, and the Center for Food Safety, went after Impossible Burger for using GMO heme and soy. They perpetuated the same fearmongering around GMO in Impossible Burgers as they had used around GMO in corn – claiming it hurt rats in a feeding study. And Impossible Food fought back, Rachel Conrad, Chief Communications officer wrote, “Finally, we’d like to request that Consumer Reports disclose its anti-GMO agenda in full transparency, and the biases of its activist employees. For years Consumers Reports, and fellow anti-GMO ideologues have been waging a PR war against GMOs — whether in vaccines, insulin, cheese or more recently the Impossible Burger.” And likewise, the PinkGlow GE pineapple that contains lycopene, a pigment that gives some produce its red color has been success, fetching a premium of as high as $50 per pineapple.
These GE applications might be considered frivolous, after all we can live without fluorescent aquarium fish and pink pineapples. But they are market successes because 1) they were allowed to come to market, and 2) they are products that the customer wanted with at least a perceived benefit. One thing is for sure – if products are not commercially available because it is cost-prohibitive, or even impossible to get regulatory approval, then the public will not be able to indicate their acceptance by purchasing them. That has essentially been the situation for GE food animals for the past 35 years (Van Eenennaam et al., 2021). And for GE food in Europe more generally, although there is of course a glaring incongruity there. In 2018 alone, the EU imported more than 30 million metric tons (MT) of soybean products, 10 to 15 million MT of corn products, and 2.5 to 4.5 million MT of rapeseed products, mainly for livestock feed. The EU’s main suppliers are Argentina, Brazil and the United States. The share of GE products of total imports is estimated at 90-95 percent for soybean products, 20-25 percent for corn, and less than 20 percent for rapeseed (USDA Foreign Agricultural Service, 2018), suggesting GMO feeds are a resounding market success! Again the lack of a requirement to label milk, meat and eggs from animals fed GE feed avoided demonization of these products and has facilitated the widespread use of GE feed in animal production systems globally.
I’ll leave you with a 60 second video of a delicious meal we prepared recently featuring the first approved GE food animal – the AquAdvantage salmon. Delicious.