Because I did not think biotechnology and hen housing were controversial enough topics, I thought I would wade into antibiotic use in food animals. Actually, the only reason I am doing this blog is because of a Twitter exchange with Marc Brazeau from Food and Farm Discussion Lab late last year. He posed several questions to me and I postponed responding unitl I had a little time to delve into the science. The answers are necessarily a bit sciency – if you want to cut to the chase, scroll directly down to Question 7.
1. How much are antibiotics used in food animal agriculture?
We have sales records from 2009-2016 from the FDA for food animal sales, but I couldn’t find any on the human side of things since April of 2012 (2011 data). The quality of the data is not great – in that it is sales data – and because some food animal products are labelled for multiple species it is a bit hard to interpret use – was that drug sold to treat a horse or a cow or a dog? The data are broken down into “medically-important” versus “not medically-important” as determined by the FDA (Appendix A).
In 2016, there were 8.36 million kg of “medically-important” and 5.62 million kg of “not medically-important” antimicrobial drugs (e.g. ionophores) sold. The good news is that these numbers represent 14% and 4% decreases, respectively, over 2015 sales data. These can be contrasted to the last data available for humans which is 3.29 million kg sold in the U.S. market in 2011. Hard to detemine the trend in human prescriptions in the absence of data.
The FDA warns that there are a number of differences in the circumstances in which antimicrobial drugs are used in human and veterinary medicine that must be carefully considered before making comparisons between human and animal use, including:
- The number of humans in the U.S. population (approx. 320 million) compared to the much, much larger number of animals in each of the many animal species (e.g., approx. 9 billion chickens slaughtered annually)
- The differences in physical characteristics of humans compared to various animal species (e.g., physiology and weight– average adult human, 182 lb vs adult cattle live weight, 1,363 lb).
- Veterinarians commonly utilize human antimicrobial drugs in their companion animal patients; therefore, amounts presented for certain human antimicrobial drugs may represent some unknown portion sold for use in companion animals. More on this point later!
According to the FDA it is, therefore “difficult to draw conclusions from any direct comparisons between the quantity of antimicrobial drugs sold for use in humans and the animal drug sales and distribution data (and species specific estimates) for use in animals.”
2. What types of antibiotics are used in animal agriculture?
It should be noted that there were some fairly sweeping changes that went into effect in 2017 as part of an effort to promote the judicious use of “medically-important” antimicrobial drugs in food animals.
The FDA has approved antibiotics for only these 3 uses in food animals:
- Disease treatment for animals that are sick;
- Disease control for a group of animals when some of the animals are sick;
- Disease prevention for animals that are at risk of becoming sick.
Animal health companies, farmers and veterinarians cooperated with the FDA to develop a guidance which ended the use of antibiotics important to human medicine to promote growth in animals or to improve feed efficiency (i.e., production purposes). The remaining therapeutic uses in feed and water are required to be under the supervision of licensed veterinarians through the Veterinary Feed Directive (VFD) (feed uses) or prescriptions (water uses) which went into effect on 1/1/2017. These changes were enacted to ensure these drugs are used judiciously and only when appropriate for specific animal health purposes.
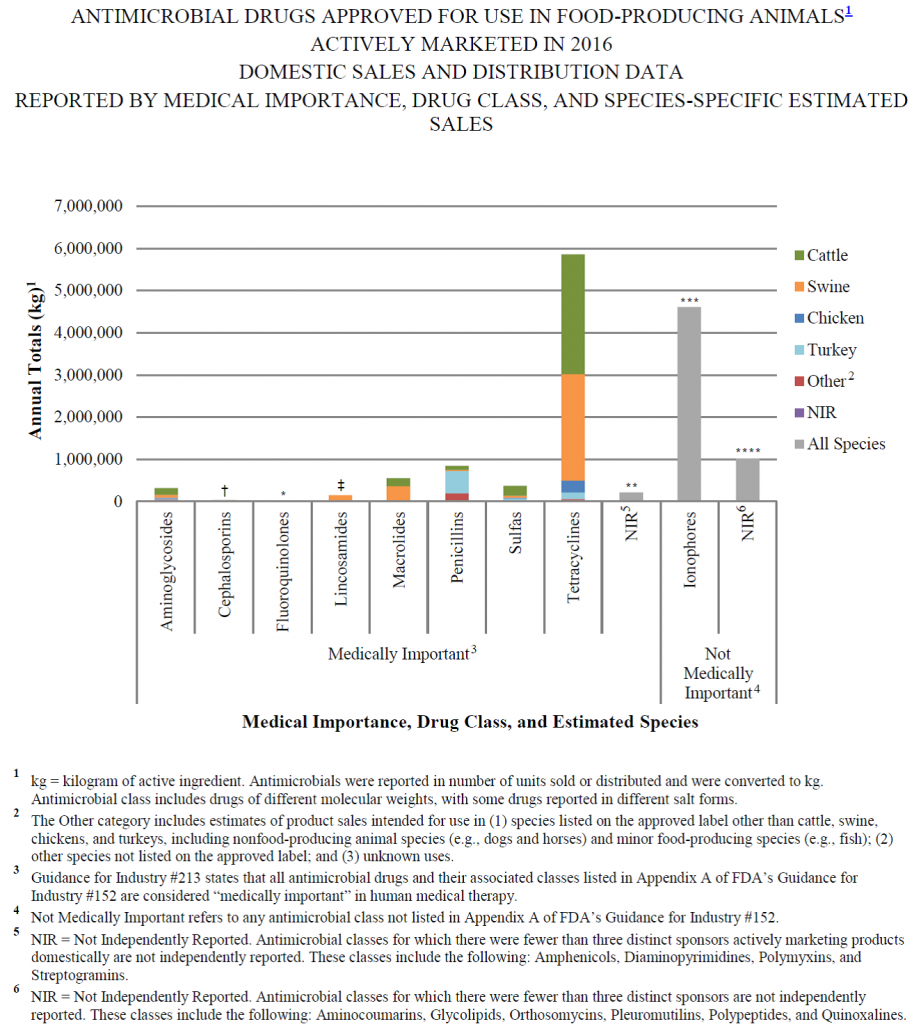
As can be seen from the graph above, by far the biggest class of antibiotics used in food-producing animals in 2016 was tetracyclines, a class of antibiotics which represented only ~ 3.5% of human antibiotic sales in 2011. This number will likely fall in 2017 as a result of the cessation of the use of “medically-important” antibiotics for production purposes.
3. What is the current state of knowledge about the transfer of resistant bacteria from livestock farms and manure use to human populations? Farm workers first and foremost.
Based on the available literature, direct transfer to humans seems to occur at a pretty minor level. One paper estimated that direct infection with resistant bacteria from an animal source, or through ingestion of bacteria from contaminated meat or water, was a relatively small risk in comparison with the overall burden of resistant disease. There was one example in the US in the past year where a Salmonella outbreak of S. heidelberg infected 54 people of whom 34 (63%) reported contact with ill calves (0 deaths). To put this in perspective, there are 1.2 million food-borne Salmonella infections per year (450 deaths). Interestingly, dogs and cats can also carry and transmit Salmonella and Campylobacter organisms, along with other pathogens traditionally associated with “foodborne diseases”. One older paper raises the question of whether dogs or other companion animals are involved in transmitting such pathogens to food-producing animals or humans, an issue which is often overlooked.

We share our homes and our microbiota with our companion animals
According to one paper examining the prevalence of within-household sharing of fecal Escherichia coli between dogs and their owners, both direct contact and environmental reservoirs were seen to be routes of cross-species sharing of bacteria and genes for resistance. The authors warned that cross-species bacterial sharing is a potential public health concern, and good hygiene is recommended (i.e. wash your hands after cleaning up after your pets!!).
It is known than human exposure to zoonotic nematodes and cestodes and other parasites associated with feces of companion animals in the United States is an ongoing public health problem. And statistically it is more likely most people will come into contact with one of the 140,000,000 dogs and cats in the US, than directly with livestock. The transmission of antimicrobial-resistant organisms between humans and pets warrants further investigation, especially as pets can be treated with “medically-important” antimicrobial drugs.
4. Is there evidence showing resistance originates in the animal population and then moves to humans?
A recent 2017 systematic review and meta-analysis to summarise the effect that interventions to reduce antibiotic use in food-producing animals have on the presence of antibiotic-resistant bacteria in animals and humans, and funded by the WHO, concluded:
“Interventions that restrict antibiotic use in food-producing animals are associated with a reduction in the presence of antibiotic-resistant bacteria in these animals. A smaller body of evidence suggests a similar association in the studied human populations, particularly those with direct exposure to food-producing animals. The implications for the general human population are less clear, given the low number of studies.
The Centers for Disease Control (CDC) issued a 2013 study on the most concerning antibiotic resistance threats and none of the most urgent threats have any relation to farm animals. On the broader CDC list, which includes less urgent threats, only two of 18 involve bacteria associated with farm animals.
A comprehensive 2016 review of 50 studies published in Critical Reviews in Food Science and Nutrition concluded that there is an established connection between animal antibiotic use and antibiotic resistance in animals, but no established causal relationship between animal antibiotic use and human resistance related to campylobacter.
There is an interesting example of an antibiotic drug called avoparcin that was used in EU agriculture for livestock growth promotion. The human equivalent is vancomycin which is used to treat E. faecium, and there was concern around vancomycin-resistant E. faecium (VRE). Avoparcin was banned in Denmark in 1997 as part of their elimination of production uses of antibiotics, and as a result VRE declined in farm animals but increased in hospitals where human vancomycin use & VRE are highly correlated. The US never approved avoparcin in livestock yet VRE started here in the 1990s due most likely to human vancomycin usage or quite possibly use in companion animals as VRE precursors have been isolated in dogs.
Likewise, this paper states that in 2005 the emergence of fluoroquinolone-resistant Campylobacter jejuni in the clinical setting in conjunction with fluoroquinolone administration in animals prompted the FDA to ban fluoroquinolone use in poultry, although it remains unclear if the dramatic increase of fluoroquinolone-resistant strains was due to fluoroquinolone use in livestock. In the USA, no decline in the levels of ciprofloxacin resistance was observed following the ban of fluoroquinolones in chickens. While it is possible that insufficient time has elapsed for trends to be detectable, it is also possible that fluoroquinolone-resistant strains may remain in the environment in the absence of antibiotic selective pressure.
On a broader scale, the EU and especially Sweden and Denmark have removed all growth promoting uses of antibiotics, and I am not familiar with any data showing this has moved the needle on resistance in human populations.
5. How reasonable is it to be concerned that bacteria resistant to an Ag-centric ABx becomes just a mutation or two away from resistance to medically important ABx with a similar mode of action.
According to the WHO-funded 2017 systematic review and meta-analysis,
“There is currently no consensus regarding the effect that antibiotic use in food-producing animals has on antibiotic resistance in the human population. Furthermore, the effect of interventions that restrict antibiotics in food-producing animals on antibiotic resistance in both animals and humans is somewhat unclear.”
In the discussion section, this 2015 paper states that,
“The topic of agricultural antibiotic use is complex. As we noted at the start, many believe that agricultural antibiotics have become a critical threat to human health. While the concern is not unwarranted, the extent of the problem may be exaggerated. There is no evidence that agriculture is ‘largely to blame’ for the increase in resistant strains and we should not be distracted from finding adequate ways to ensure appropriate antibiotic use in all settings, the most important of which being clinical medicine.”
You can be worried about it, but I think the objective data is much stronger on resistance coming for the most part by way of human medicine use. Anytime antibiotics are used they will lead to resistance – whether used to treat livestock, dogs or people.
6 . What are the recommendations of the World Health Organization Guidelines on use of medically important antimicrobials in food-producing animals ?
The first three recommendations of the WHO guidelines are based on “low quality evidence”
- We recommend an overall reduction in use of all classes of medically important antimicrobials in food-producing animals.
- We recommend complete restriction of use of all classes of medically important antimicrobials in food-producing animals for growth promotion.
- We recommend complete restriction of use of all classes of medically important antimicrobials in food-producing animals for prevention of infectious diseases that have not yet been clinically diagnosed.
And the last two are conditional recommendations based on “very low quality evidence”
Recommendation: Control and treatment use (in the presence of disease)
4a. Recommendation: We suggest that antimicrobials classified as critically important for human medicine should not be used for control of the dissemination of a clinically diagnosed infectious disease identified within a group of food-producing animals.
4b. Recommendation: We suggest that antimicrobials classified as highest priority critically important for human medicine should not be used for treatment of food-producing animals with a clinically diagnosed infectious disease.
The fact that the WHO acknowledges there is low or very low quality evidence to support these apparently logical recommendations, does suggest that there is little data on the transfer of resistant bacteria from livestock farms. Irrespective, the first recommendation for an overall reduction in use of all classes of medically important antimicrobials in food-producing animals to me lacks the required nuance when dealing with disease. A blanket reduction (as in just decreasing the physical mass or amount) of antibiotics used in food animals does not necessarily mean improvement.
For example, replacing an effective antimicrobial with an ineffective antimicrobial that is used at a lower dose would result in an overall reduction in the use of antibiotics, but not with the desired effect as the animal would still be sick. And some “medically-important” antibiotics are more important/critical than others. It is analogous to the comparison that is sometimes made that the weight of glyphosate herbicide use has gone up in recent years, but that has to be looked at in terms of the effectiveness and weight and toxicity of alternative herbicides that were used to control the weed problem. Same thing here – what are alternatives for treatment and how effective are they (i.e. weigh up pros and cons and determine the most judicious choice of treatment)?
Recommendations 4a and 4b are based on very low evidence. The report states that when a veterinarian is faced with treating a clinically diagnosed infectious disease in food animal(s), “The GDG [Guideline Development Group] concluded that although evidence from the systematic reviews and additional studies indicates it will achieve the human health benefit of lowered antimicrobial resistance in bacteria, this recommendation should be conditional due to the very low quality of available evidence. ….Furthermore, the undesirable consequences associated with such a restriction of use of antimicrobials appear to be relatively small or non-existent. Finally, several countries have successfully accomplished such a restriction of antimicrobials in food-producing animals, demonstrating its feasibility.”
The second to last sentence seems rather callous in its regard for the sick animal and is particularly worrying from an animal welfare perspective. There is a remark in the guidance that states,
“To prevent harm to animal health and welfare, exceptions to recommendations 4a and 4b can be made when, in the judgment of veterinary professionals, bacterial culture and sensitivity results demonstrate that the selected drug is the only treatment option.”
To my knowledge there is no country that does not allow treatment of animals with a clinically diagnosed infectious disease. And so one wonders where that leaves veterinarians when their only option is a drug that is critically important to human medicine – do they use avoid using “antimicrobials classified as highest priority critically important for human medicine” based on very low quality evidence or do they use them to prevent harm to animal health and welfare?
Some of the replacements of antibiotics that have been used in Europe are themselves associated with their own set of problems, for example the application of zinc oxide has been a key alternative to the reduction of antibiotics usage in Sweden and Denmark. The European Commission, however, has pointed to zinc oxide as having a serious impact on the environment as much of the substance gets excreted and ends up in fields when the manure is applied on the lands. In some studies, the use of zinc oxide has been associated with the occurrence of methicillin-resistant Staphylococcus aureus (MRSA) as the resistant bacteria might carry zinc-resistance genes. That is, as always, tradeoffs associated with different choices and the antibiotic replacements are not without their own set of risks and tradeoffs.
7. I don’t care – I just want food animals never to get treated with antibiotics irrespective
We still don’t have strong evidence linking animal use with antibiotic resistance in the human population. Antibiotics along with other management and health factors go into animal welfare – if we can’t use them animals will get sicker and this can affect One Health (the unity of multiple practices that work together locally, nationally, and globally to help achieve optimal health for people, animals, and the environment) goals.
For example, the “no antibiotics ever” (never ever) marketing campaign may actually put the food supply at risk. If there is more untreated infectious disease, then pathogen prevalence could increase and this would increase the pathogen load of the raw product coming into plant which could affect food safety from the perspective of food-borne pathogens.
And more generally is the question of what should be done with sick animals then? At the moment there are three choices:
- treat the animal(s) and dump the animals into someone else’s supply chain
- leave untreated and sell the ones that survive which seems rather callous and Darwinian
- euthanize sick animals (including entire flocks) which comes with its own set of sustainability issues

Dairy Heifer with a bad case of pinkeye and blindness in that eye in a production system that prohibits the use of antibiotics for treatment of the bacteria which cause that disease
There is an understanding that this is an important issue and the industry is working to address it but I personally think there is both a One Health and welfare need to keep access to strategic uses. The dairy heifer in the picture above has a raging pink eye in her right eye as you can see and was on a “never ever” antibiotic farm in Northern California – so she never ever got antibiotics and her eye just blew up and developed a perforated ulcer. At the time the picture was taken, she was 14 months away from producing milk. Personally I will take the conventional milk that allowed her to get treatment in the same way I treated my own kid’s pink eye with antibiotics.
If the entire food supply chain mandated never ever treatment regimen, there would be a real welfare dilemma in terms of what to do with sick animals. At the current time the conventional supply chain takes in the “rejects” (i.e. sick animals that needed to be treated with antibiotics) of the never ever supply chain. To me there is something just not right about a system that depends, in fact relies, upon the fact that someone else’s customers will consume their rejects so that their value-added (i.e. more expensive) product can carry an absence label for what is an essential tool in their own production system. It is a bit like the EU rejecting the cultivation of GMO crops and then importing GMO soybean and corn grown in other countries to feed their livestock populations – exporting the problem to someone else’s backyard does not solve the problem. And pretending that sick animals don’t exist does not address this problem either. Our food animals deserve a more honest and transparent discussion of this topic.
